Researchers Discover How Smartwatches Can Stop Disease Spread by Early Detection


Research
Researchers Discover How Smartwatches Can Stop Disease Spread by Early Detection

Researchers from the Department of Industrial Engineering at TAU's Faculty of Engineering led a two-year study in which participants wore smartwatches that measured biomarkers and answered questions about their health every day. The results indicate that the wearable technology identified a change in key physiological parameters one to three whole days before the user felt the first symptom of the disease: a gap of 23 hours for COVID-19, 62 hours for group A streptococcus (GAS), and 73 hours for influenza.
The researchers: "Early diagnosis enabled by wearable technologies can be critical for inducing behavioral changes, such as reduced social contacts at an early stage, when the disease is most infectious. Potentially, this can prevent the spread of disease and even preempt global pandemics in the future".
The study was led by Prof. Dan Yamin, an expert in epidemiology and infectious disease modeling and Head of the Lab for Digital Epidemiology and Health Analytics, and Prof. Erez Shmueli, Head of the Big Data Lab, both from TAU's Department of Industrial Engineering. Other participants included: research students Shachar Snir and Matan Yechezkel from the Department of Industrial Engineering, Dr. Tal Patalon from the Kahn Sagol Maccabi Research and Innovation Center at Maccabi Healthcare Services and Yupeng Chen and Prof. Margaret Brandeau from the Department of Management Science and Engineering at Stanford University. The paper was published in Lancet Regional Health Europe.
Prof. Yamin: "Infectious diseases and pandemics pose a great threat to humanity, and we must harness our scientific and technological abilities to prevent them. Previous studies have shown that during the recent pandemic about 40% of all transmissions occurred about a day before the first symptoms appeared. In other words, the person transmitting the disease was unaware they were infected. In this study we checked whether wearable technologies could provide earlier diagnosis, to reduce contagion and prevent the spread of infectious diseases".
Tracking Key Health Changes
During the two-year study, 4,795 Israelis over 18 years of age wore a smartwatch that continuously monitored key physiological parameters, focusing on pulse rate at a 15-second resolution and HRV (Heart Rate Variability). Prof. Yamin explains: "Pulse rate and HRV provide crucial information about the two most important systems in our body – the heart and the brain. Our brain constantly consumes energy, burning oxygen provided by the cardiovascular system, and consequently, any change in our activity or condition is immediately reflected in a change in HRV. When a person becomes ill, most of the focus goes to a single system - the immune system battling the disease, keeping the heart rate relatively steady, and reducing its variability, the HRV. In this way, changes in HRV indicate physical stress".
In addition to wearing the smartwatches, participants answered a series of general questions about their condition every day: How do you feel physically? How do you feel mentally? Have you engaged in physical activity? Do you have any specific symptoms? Etc. In addition, they were provided with home test kits for three different diseases - COVID-19, influenza, and group A streptococcus – which they used at their discretion. Over two years, the researchers collected 800,000 questionnaires and this data was compared with parallel data from the smartwatch. Altogether, the data included 490 episodes of influenza, 2206 episodes of COVID-19, and 320 episodes of GAS.
Based on their abundant data, the researchers built special models that identified three critical points in time following exposure to an infectious disease. For instance, COVID-19: A. The first physiological anomaly in heart rate measures - 96 hours after exposure, an interval, which the researchers call the 'digital incubation period'; B. The first symptom noticed by the person –130 hours after exposure, an interval commonly known as the 'incubation period'; and C. Testing that ultimately diagnosed the disease - usually about 168 hours after exposure, called the 'diagnostic decision period'. The period from exposure to digital diagnosis, namely the digital incubation period, was even shorter for influenza (24 hours) and GAS (60 hours).
Getting Ahead of the Curve?
Prof. Shmueli: "Early diagnosis is extremely important for preventing the spread of the disease. Moreover, we found that even when our subjects reported first symptoms, they tended to postpone testing for a while - 53 hours for COVID-19, 39 hours for influenza, and 38 hours for GAS. Consequently, for quite a long interval, from exposure to testing, they did not change their social behavior, spreading the disease to others. We found that on average, people performed the test and changed their behavior when the disease was already past its peak, and they were much less likely to infect others. The delay between digital diagnosis and testing – 64 hours in the case of COVID-19, 68 hours for influenza, and 58 hours for GAS – is thus extremely crucial".

Prof. Yamin: "Our findings indicate that at the population level digital diagnosis can significantly reduce the spread of infectious diseases, by causing people to change their social behavior at a much earlier stage of the disease. This can even prevent the next pandemic – by bringing the basic reproduction number (R0value) to below 1.0, which means that every sick individual transmits the disease to less than one other person, and the disease soon dies out".
The researchers add that early diagnosis is also critical for effective treatment. Specifically, for COVID-19, existing treatments are very effective only when given early on, preventing severe illness, hospitalization, and even death.
A Milestone in Stopping Pandemics
Prof. Yamin: "In an ERC-funded paper published in October 2019, shortly before the outbreak of the COVID-19 pandemic, I argued that infectious diseases pose the greatest threat of a global catastrophe. The threat is especially great in the modern world, with people traveling all over the globe and potentially spreading new diseases. However, modern technology can help us combat this danger and devise more effective public health strategies. Our new method, using wearable sensors for early detection of contagious disease can potentially reduce the threat of epidemics to a minimum. Smartwatches are a relatively new technology, with enormous potential, and novel, even more sensitive and accurate wearable sensors are constantly being developed. Ultimately, this can be a high-impact tool for preempting future pandemics".

Research
TAU research paves the way to brain healing with parasites

Have you ever imagined that parasites could be beneficial for brain diseases? TAU Researchers have reengineered Toxoplasma gondii, the 'cat parasite,' transforming it from a feared threat into a groundbreaking tool for delivering drugs directly to the brain. This surprising innovation not only overturns our expectations but also opens new possibilities for treating neurological disorders.
In a breakthrough study by an international team of scientists led by researchers from Tel Aviv and Glasgow Universities, the 'cat parasite' Toxoplasma gondii was engineered to deliver drugs to the human brain. The study was led by Prof. Oded Rechavi from the Department of Neurobiology and the Sagol School of Neuroscience at Tel Aviv University, together with his PhD student Dr. Shahar Bracha, and with Prof. Lilach Sheiner, an Israeli scientist and toxoplasma expert from the University of Glasgow in Scotland. The results were published in the leading scientific journal Nature Microbiology.
"One of the biggest challenges in treating neurological diseases is getting through the blood-brain barrier (BBB)," explains Prof. Rechavi. "It is tough to deliver drugs to the brain via the bloodstream, and this is especially true for large molecules such as proteins, the critical 'machines' that carry out many important functions inside the cell".
Toxoplasma gondii - the 'cat parasite'
The creative solution proposed by the TAU team utilizes the unicellular parasite Toxoplasma gondii, which can infect a vast variety of organisms, but reproduces only in the guts of cats. The parasite is very effective in infecting humans, with an estimated third of the global population infected at some point in their lives. Prof. Rechavi explains: "Most people don't even feel the infection or only experience mild flu-like symptoms.
The parasite is, however, dangerous for people with immune failure due to conditions like AIDS, and for fetuses whose immune system has not yet developed. This is why pregnant women are advised not to eat raw meat which might contain the parasite, and to stay away from cats, which might deliver it through their feces. While ridding the body of the parasite, a healthy immune system has only limited access to the brain, and the parasite remains in the brain throughout the carrier's lifetime".
The parasite's ability to penetrate the human brain and survive there in a dormant state, without reproducing, made it a perfect candidate for the researchers' novel approach: genetically engineering Toxoplasma gondii to secrete therapeutic proteins.
"The parasite has three distinct secretion systems and we 'hitched a ride' on two of them", says Prof. Rechavi. "We did not intervene with the first system, which secretes proteins outside the neurons. The second system 'shoots' a 'harpoon' into the neuron, to enable penetration. Once inside, the parasite forms a kind of cyst that continues to secrete proteins permanently. We engineered the parasite's DNA to make it produce and secrete the proteins we want, which have therapeutic potential".
"The parasite's ability to pass through the BBB and communicate with the neurons, combined with our ability to engineer the parasite, generate a golden opportunity for solving the great therapeutic challenge of delivering medications to the brain", says Prof. Sheiner.

Illustration of the activity of neurons
In this study, the team used transgenic model animals that were injected with parasites genetically engineered to produce and secrete proteins that travel into cell nuclei. Several lines of evidence proved that the proteins had been delivered to the target area and remained active in the neurons' nuclei. One of these was especially eye-catching: a protein that, delivered by the parasite, entered the nuclei and cut out specific DNA segments, causing the transgenic animals’ brains to glow in the dark.
This breakthrough can have far-reaching implications for a series of severe diseases. In the present study, the researchers specifically demonstrated the delivery of a protein called MeCP2, whose deficiency is associated with Rett syndrome. "This is a deadly syndrome caused by a deficiency in a single gene called MePC2 in brain cells, and our engineered Toxoplasma gondii was able to deliver it to the target cells", says Prof. Rechavi. "But this is just one example. There are many other diseases caused by deficiency or abnormal expression of a certain protein". To ensure the method's safe and effective therapeutic implementation, for both drug delivery and genetic editing, a company named Epeius was established in collaboration with Ramot – the technology transfer company of Tel Aviv University, and with the University of Glasgow's research and innovation services.

Research
TAU researchers discover that bats have episodic memory and plan ahead

Researchers at Tel Aviv University tracked free-ranging Egyptian fruit bats from a colony based in the TAU’s I. Meier Segals Garden for Zoological Research to answer a long-standing scientific question: Do animals have high and complex cognitive abilities, previously attributed only to humans? In particular, the study focused on the traits of episodic memory, mental time travel, planning ahead, and delayed gratification, arriving at highly thought-provoking conclusions.
The study was led by Prof. Yossi Yovel and Dr. Lee Harten from the School of Zoology and Sagol School of Neuroscience at Tel Aviv University. Other researchers included: Xing Chen, Adi Rachum, Michal Handel, and Aya Goldstein from the School of Zoology, Lior de Marcas from the Sagol School of Neuroscience, and Maya Fenigstein Levi and Shira Rosencwaig from the National Public Health Laboratory of Israel's Ministry of Health. The paper was published in Current Biology.

Prof. Yossi Yovel.
2021 Kadar Family Award recipient Prof. Yovel: "For many years the cognitive abilities to recall personal experiences (episodic memory) and plan ahead were considered exclusive to humans. But more and more studies have suggested that various animals also possess such capabilities. Still, nearly all of these studies were conducted under laboratory conditions, since field studies on these issues are difficult to perform. Attempting to test these abilities in wild animals, we designed a unique experiment relying on the colony of free-ranging fruit bats based in TAU’s I. Meier Segals Garden for Zoological Research".
How Bats Keep Track of Food Resources
The researchers assumed that bats depending on fruit trees for their survival would need to develop an ability to track the availability of food both spatially (where are the fruit trees?) and over time (when does each tree give fruit?). Navigating through landscapes with numerous fruit and nectar trees, they would need to mentally track the resources in order to revisit them at the appropriate time. To test this hypothesis, a tiny high-resolution GPS tracker was attached to each bat, enabling the documentation of flight routes and trees visited for many months. The vast data collected in this way were thoroughly analyzed, producing some amazing results.
The first research question was: Do bats form a time map in their minds? To explore this issue, the researchers prevented the bats from leaving the colony for varying periods of time, from one day to a week. Dr. Harten: "We wanted to see whether the bats could tell that time had elapsed and behave accordingly. We found that after one day of captivity, the bats would return to trees visited on the previous night. However, when a whole week had gone by, the older bats, based on past experience, avoided trees that had stopped bearing fruit in the interval. In other words: they were able to estimate how much time had passed since their last visit to each tree and knew which trees bore fruit for a short time and were no longer worth visiting. Young, inexperienced bats were unable to do this, indicating that this is an acquired skill that must be learned".

While the first research question looked at past experiences, the second dealt with the future: Do the bats exhibit future-oriented behaviors? Are they capable of planning ahead? To address this issue the researchers observed each bat's route to the first tree of the evening, possibly indicative of plans made before leaving the colony. Chen Xing: "We found that usually the bats fly directly to a specific tree they know, sometimes 20 or 30 minutes away. Being hungry, they fly faster when that tree is further away, suggesting they plan where they are heading.
Moreover, focused on their chosen target, they will pass by other trees, even good sources visited just yesterday – indicating a capacity for delayed gratification. We also found that the first bats to leave the colony choose trees bearing fruits rich in sugar, while the bats that leave later seek proteins." All these findings suggest that the bats plan their foraging before they leave the colony, and know exactly where they are flying and what kind of nourishment they are looking for.
Rethinking Intelligence in Animals
Prof. Yovel: "The cognitive gap between humans and animals is one of the most fascinating issues in science. Our study demonstrates that fruit bats are capable of quite a complex decision-making process involving the three questions indicative of cognitive abilities: Where? (each tree's location); When? (when the tree bears fruit); and What? (the nourishment it provides – sugar vs. proteins). Once again we find that the gap is not cleat-cut, and that humans are not as unique as some might think. Apparently, humans and animals are all located on a spectrum, with almost any human ability found in animals as well".

Research
TAU research introduces smart tagging to identify and track drones in extreme weather conditions

A new development by researchers at the Faculty of Engineering at Tel Aviv University will help identify small drones in challenging scenarios, such as urban environments, low flight altitudes, and extreme weather conditions, enhancing the protection of airspaces via smart tagging. The research team notes that drone identification is generally conducted using radars, cameras, and transponders, with the latter providing real-time updates on location in civilian contexts. However, these methods can fail in harsh conditions, including limited line of sight, multiple air traffic participants, and tall buildings blocking satellite signals, among other challenges.
The researchers highlight that this new technology can overcome these challenges and provide a superior level of reliability by using smart stickers and a radar supported by an AI algorithm that classifies drones based on the electromagnetic radiation they scatter.
The development was led by Ph.D. students Omer Tzidki and Dmytro Vovchuk from Prof. Pavel Ginzburg’s lab, the Iby and Aladar Fleischman Faculty of Engineering. The lab specializes in developing novel radar and wireless communication technologies, facing new and forthcoming challenges.
Omer Tzidki points out that the problem of identifying the drones is especially critical when there is no direct line of sight, for example when the drone is hidden behind a cloud, in fog, or hard to see due to adverse weather conditions. In these situations, cameras alone are insufficient, and the use of radar becomes necessary.
With this new development, identification is carried out through an electromagnetic representation of the drone’s "identity card". This allows the radar to distinguish between drones with different IDs by using electromagnetic tagging on the drone’s wings. The AI algorithm, which relies on a neural network, classifies the drone as either friendly or hostile and operates successfully even in varying harsh conditions while minimizing the risk of accidents. Initial experiments were conducted under laboratory conditions in a sterile environment, followed by trials in an external setting to simulate real-world scenarios.
2025 Kadar Family Award recipient Prof. Pavel Ginzburg: "The simplest things often work best. This project leverages fundamental physical principles to reliably and accurately classify drones. The process of identifying any drone using radar is quite complex, so achieving the capability to identify specific drones is a significant accomplishment of which we are very proud".
Omer Tzidki emphasizes that the combination of electromagnetic techniques, AI algorithms, and innovative radar technology yields optimal results. "Mapping the airfield is critical for protecting the lives of soldiers and civilians. This project is important at all times and especially crucial now", he said.

Research
Researchers achieve success in allowing a patient to “speak” using only the power of thought

A scientific breakthrough by researchers from Tel Aviv University and Tel Aviv Sourasky Medical Center (Ichilov Hospital) has demonstrated the potential for speech by a silent person using the power of thought only. In an experiment, a silent participant imagined saying one of two syllables. Depth electrodes implanted in his brain transmitted the electrical signals to a computer, which then vocalized the syllables.
The study was led by Dr. Ariel Tankus of Tel Aviv University’s School of Medical and Health Sciences and Tel Aviv Sourasky Medical Center (Ichilov Hospital), along with Dr. Ido Strauss of Tel Aviv University’s School of Medical and Health Sciences and director of the Functional Neurosurgery Unit at Ichilov Hospital. The results of this groundbreaking study were published in the prestigious journal Neurosurgery, the official publication of the Congress of Neurological Surgeons. These findings offer hope for enabling people who are completely paralyzed — due to conditions such as ALS, brainstem stroke, or brain injury — to regain the ability to speak voluntarily.

Dr. Ariel Tankus of Tel Aviv University’s School of Medical and Health Sciences and Tel Aviv Sourasky Medical Center (Ichilov Hospital).

Dr. Ido Strauss of Tel Aviv University’s School of Medical and Health Sciences and director of the Functional Neurosurgery Unit at Ichilov Hospital. Photo credit: Lior Zur, Tel Aviv Sourasky Medical Center (Ichilov Hospital).
"The patient in the study is an epilepsy patient who was hospitalized to undergo resection of the epileptic focus in his brain", explains Dr. Tankus. "to do this, of course, you need to locate the focal point, which is the source of the ‘short’ that sends powerful electrical waves through the brain. This situation pertains to a smaller subset of epilepsy patients who do not respond well to medication and require neurosurgical intervention, and an even smaller subset of epilepsy patients whose suspected focus is located deep within the brain, rather than on the surface of the cortex. To identify the exact location, electrodes have to be implanted into deep structures of their brains. They are then hospitalized, awaiting the next seizure. When a seizure occurs, the electrodes will tell the neurologists and neurosurgeons where the focus is, allowing them to operate precisely. From a scientific perspective, this provides a rare opportunity to get a glimpse into the depths of a living human brain. Fortunately, the epilepsy patient hospitalized at Ichilov agreed to participate in the experiment, which may ultimately help completely paralyzed individuals to express themselves again through artificial speech".
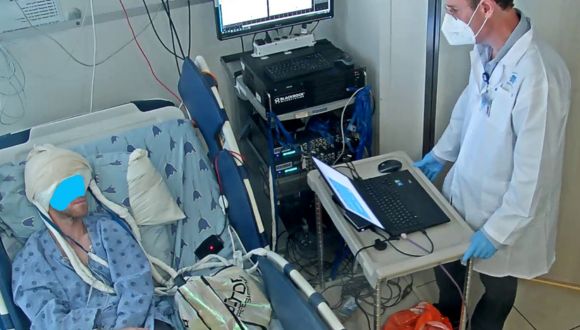
An image from the experiment of the speech neuroprosthesis (a.k.a speech brain-computer interface). It shows the participant who is completely silent, with his mouth closed, imagining saying a syllable. The laptop "says" the syllable for him.
In the first stage of the experiment, with the depth electrodes already implanted in the patient’s brain, the Tel Aviv University researchers asked him to say two syllables out loud: /a/ and /e/. They recorded the brain activity as he articulated these sounds. Using deep learning and machine learning, the researchers trained artificial intelligence models to identify the specific brain cells whose electrical activity indicated the desire to say /a/ or /e/. Once the computer learned to recognize the pattern of electrical activity associated with these two syllables in the patient's brain, he was asked only to imagine that he was saying /a/ and /e/. The computer then translated the electrical signals and played the pre-recorded sounds of /a/ or /e/ accordingly.
"My field of research deals with the encoding and decoding of speech, that is, how individual brain cells participate in the speech process — the production of speech, the hearing of speech, and the imagination of speech, or ‘speaking silently", says Dr. Tankus. "In this experiment, for the first time in history, we were able to connect the parts of speech to the activity of individual cells from the regions of the brain from which we recorded. This allowed us to distinguish between the electrical signals that represent the sounds /a/ and /e/. At the moment, our research involves two building blocks of speech, two syllables. Of course, our ambition is to get to complete speech, but even two different syllables can enable a fully paralyzed person to signal ‘yes' and ‘no.’ For example, in the future, it will be possible to train a computer for an ALS patient in the early stages of the disease, while they can still speak. The computer would learn to recognize the electrical signals in the patient’s brain, enabling it to interpret these signals even after they lose the ability to move their muscles. And that is just one example. Our study is a significant step toward developing a brain-computer interface that can replace the brain's control pathways for speech production, allowing completely paralyzed individuals to communicate voluntarily with their surroundings once again".
The study was supported by a grant from the Israel Ministry of Innovation, Science and Technology.