Imagining the Future Patient
Biomedical and technological progress is at the tipping point of transforming medicine into a more precise, proactive science capable of defeating human disease
The 21st century is revolutionizing our approach to healthcare, our understanding of the human body, and our ability to intervene in its most intricate processes. Tel Aviv University researchers are at the forefront of the advancing changes. They are collaborating with Israel’s hospitals and industry to compute a patient’s future, popularize genetic screenings, develop novel vaccines, and usher in the era of truly personalized treatment.
Transforming Sick Care into Health Care
The last decade has seen an explosion in the amount of electronic medical records. Around the world, massive databases containing comprehensive genetic and health information on hundreds of millions of people have been collected at hospitals, clinics, and data repositories. Alongside it, the revolution in AI is enabling the development of computational tools to accurately analyze this data on an unprecedented scale. At this intersection, the field of bioinformatics, which aims to solve biomedical problems by using computer science tools, is becoming increasingly important in transforming medicine from a reactive to a proactive science.
“It became obvious very fast that analyzing this data would provide fantastic insights to understanding how disease transpires and progresses, and to offer novel approaches to diagnosis, treatment, and prevention,” says Ron Shamir, Professor Emeritus of the Blavatnik School of Computer Science at the Raymond and Beverly Sackler Faculty of Exact Sciences and the founding director of TAU’s Edmond J. Safra Center for Bioinformatics and Koret-Berkeley-TAU Initiative in Computational Biology and Bioinformatics. The Edmond J. Safra Center for Bioinformatics brings together all bioinformatics-related research and teaching activities across campus into one multidisciplinary hub, spanning over 50 research groups and 200 students across four faculties.
“A single individual’s genome is three billion letters. Working with this amount of data feels like drinking from a fire hydrant at times” – Professor Emeritus Ron Shamir
Navigating the Data Labyrinth
Data is the backbone of all bioinformatics research but it’s not an easy work partner—sometimes its sheer amount and complexity are overwhelming. “A single individual’s genome is three billion letters. Working with this amount of data feels like drinking from a fire hydrant at times,” Shamir says.
To assist TAU researchers in addressing this problem and questions of data privacy and security, TAU recently established the Health Data Science Hub — a joint unit of the Edmond J. Safra Center for Bioinformatics, TAU’s AI & Data Science Center, the Faculty of Medicine, and the Biomedical Engineering Department at The Iby and Aladar Fleischman Faculty of Engineering.
“The Hub will be the center of knowledge and expertise on the various protocols needed to access large data repositories and will streamline the process for TAU scientists, which will be a great help in facilitating research,” explains current Head of Edmond J. Safra Center for Bioinformatics, Prof. Elhanan Borenstein, of the Blavatnik School of Computer Sciences and the medical faculty.
“To advance the future of medicine, we need to broaden the set of tools physicians use to understand a patient’s medical situation. Researchers and physicians need to talk to each other.” - Prof. Elhanan Borenstein
Physicians of the Future
Another long-term mission of TAU’s bioinformatic programs is to educate physicians about the potential of digital medicine.
To achieve this, TAU introduced several novel study programs in the 2022-23 academic year. First, a joint Bioinformatics-MD degree, which will arm future doctors with advanced data crunching skills. Another is a Big Data in Healthcare course offered by the Faculty of Medicine in collaboration with a governmental and industry consortium called the “8400 Health Network.” The course allows participants, many of whom are practicing physicians, to become acquainted with the opportunities now available to them in the digital healthcare realm. A total of 480 candidates applied for the 80 spots available in the course pilot.
“To advance the future of medicine, we need to broaden the set of tools physicians use to understand a patient’s medical situation. Researchers and physicians need to talk to each other,” Borenstein explains. To facilitate this, the Edmond J. Safra Center for Bioinformatics spearheads dozens of collaborative projects in clinical bioinformatics with various hospitals, programs, and academic institutions. It awarded 22 grants in the last two years for joint research projects, and an additional five collaborative grants are expected to be awarded in the coming months.
Preventive Genetics
One example of such a collaboration is Prof. Ran Elkon’s lab at the Department of Human Molecular Genetics and Biochemistry, Faculty of Medicine. Elkon’s work centers on understanding the genetic basis of widespread complex diseases, such as high blood pressure, stroke, cancer, cardiovascular diseases, diabetes, and even mental illnesses.

Prof. Ran Elkon
“These diseases are not considered genetic as the term is commonly understood, but it’s clear that they have a genetic component, which we call ‘predisposition,’” says Elkon. Rather than single-gene mutations, hundreds of small genetic variations influence the risk of contracting such diseases, he explains. “Each individual variant has very little effect on its own; what matters is the collective amount of such ‘risk variants’ in each individual genome.”
Elkon’s lab team recently completed a project on identifying women with an elevated genetic predisposition to breast cancer and showed that findings are applicable in Israel. Elkon is now working with Israel’s largest HMO, Clalit, to launch a clinical study for identifying such women among Clalit’s clients and offering them a more personalized breast cancer screening strategy.
“With genetic testing, we can develop more personalized, more precise approaches that will be much more effective for prevention and early detection of [breast cancer].” - Prof. Ran Elkon
The likelihood of developing breast cancer is 16 times higher for women in the top 1% genetic risk group compared to those in a low-risk group. However, today in the developed world, healthcare providers offer identical screening recommendations and coverage for women on both ends of the spectrum, Elkon explains. “We are trying to change this one-size-fits-all strategy on the ground. With genetic testing, we can develop more personalized, more precise approaches that will be much more effective for prevention and early detection of the disease,” he says.
Elkon also places a major focus on teaching ‘predictive genetics’ in his genetics classes for TAU medical students. “Genetic screening will become common practice, similar to routine blood work and other widespread check-ups, and future physicians need to be aware of this. Early detection has a major effect on prognosis and survival,” he explains.
Prof. Elkon works on trying to solve the genetic inequality dilemma, which has surfaced alongside progress in the field. Of all massive genetic breast cancer studies in the world, 90% were conducted on women of European ancestry, making findings and discoveries relevant mostly to this ethnicity. Elkon and his team were able to show that while in Israel the findings translate well to women of Ashkenazi descent, predictive performance substantively declines for individuals of other ethnicities, such as North African, Ethiopian or Druze. Elkon hopes to help come up with computational tools to successfully transfer the findings cross-ethnically. Together with physicians at the Rabin Medical Center (Beilinson Hospital) and the Clalit HMO, he’s heading research on the topic.
"We can turn a person’s skin cell into an undifferentiated stem cell, and from there, into a cell of any organ we want.” – Dr. Ben Maoz
Treatment Personalization
Prevention is the ideal alternative, but what can be done about treatment when disease does occur? On the other side of campus, in his cutting-edge lab at the Susan and Henry Samueli Engineering Building, Dr. Ben Maoz of the Fleischman Faculty of Engineering and the Sagol School of Neuroscience is revolutionizing drug development and treatment personalization.
“The drug development process hasn’t changed in 70 years. It takes a lot of time – about 20 years and $2 billion to develop an FDA-approved medication. And even then, once the drug has been approved, it is not optimal for about 75% of the people taking it, because we all have unique physiologies,” he explains.
Maoz is developing the ‘Organs-on-a-Chip’ technology that circumvents the traditional need for animal drug trials and lets researchers test new medication on something much more similar to humans than rodents—human organ models made of lab-grown cells.
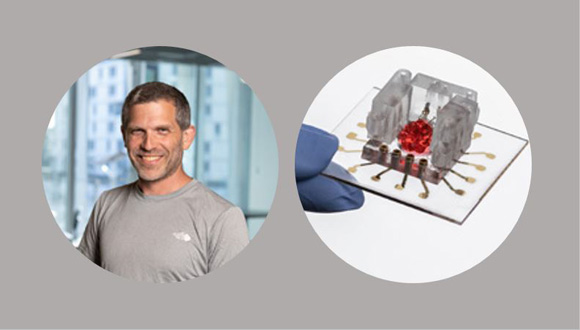
Dr. Ben Maoz and his 'Organ-on-a-Chip' model
“It’s a process that takes up to four months overall. We can turn a person’s skin cell into an undifferentiated stem cell, and from there, into a cell of any organ we want,” Maoz explains. Once the researchers have the desired tissue with the specific DNA content they cast it into lego-like units which can be interconnected to mimic the complex physiological system of a specific patient. There is no limit on how many times this can be replicated for multiple trial-and-error runs.
“Parallel trials are especially important when there are multiple treatment options and, without personalized data, we just don’t know which one will work better. In the case of diseases such as cancer, that is crucial information,” he says.
Recently, in a move that exceeded expectations, the FDA approved the Organs-on-a-Chip approach to serve as a complementary tool for drug development, eliminating the unconditional need for animal trials. “This is a major step forward — it opens the door for expediting drug development and making it much more efficient and personalized.”
Maoz’s lab currently collaborates with numerous scientists and hospitals in Israel and the world, as well as three pharmaceutical companies that wish to use the TAU technology to test their drugs for toxicity and efficacy.
While many improvements are still needed, Maoz is certain the technology is here to stay. As for scalability, he says insurance companies will understand that channeling resources into focused, personalized testing and optimal solutions, instead of spending money on ineffective treatments and procedures, is a better and more financially viable strategy. “In the future, patients will arrive, create ‘mini-me’s-on-a-chip’ in fully robotic laboratories, and get an optimal drug for their condition,” Maoz concludes.
Harvesting Stem Cells
Recognizing the increasing demand for cellular material derived from stem cells, and addressing the lack of such facilities in Israel, TAU recently established the Stem Cell Core Lab for Regenerative Medicine, a multidisciplinary initiative of the Faculties of Medicine, Engineering, and The George S. Wise Faculty of Life Sciences; the Sagol Center for Regenerative Medicine; and TAU’s Vice President for R&D.
The lab already serves dozens of research groups across the TAU campus, as well as from other Israeli research institutions and commercial companies. Research based on stem cell technology advances exciting scientific breakthroughs in precision medicine and the Center is there “to help it reach stages of early clinical evaluation.”
Gene Therapy for All
"In gene therapy, the 'one-size-fits-all' approach often works and may even be the preferred option," says Dr. Adi Barzel from the School of Neurobiology, Biochemistry & Biophysics at the George S. Wise Faculty of Life Sciences.

Dr. Adi Barzel (center) with Dr. Erik Shifrut and Dr. Anat Globerson Levin at their lab in the Sourasky Medical Center (Ichilov Hospital).
“The COVID vaccines, several cancer therapy drugs, and therapies for rare diseases, which are all already FDA-approved and used on the market, are examples of generic gene therapies that work for most,” he explains.
"Gene therapy is no longer a dream; it is a reality." - Dr. Adi Barzel
Barzel’s team works on engineering the cells of the human immune system so that they can better combat cancer, infections, and autoimmune diseases. Last year, in a world first, they succeeded in using the gene-editing technology CRISPR to engineer type-B white blood cells with antibodies to successfully fight the HIV virus.
“For this specific drug it will take another 5 to 6 years before we get to clinical trials — these technologies take time to mature, but gene therapy is no longer a dream, it is a reality,” Barzel states.
Barzel envisions that similar to the COVID vaccine, the HIV medication would need no personal adjustment, and ideally be available “in vials at your neighborhood clinic”, and affordable to all.
He predicts that in 10 years we will see many more treatments based on genome editing, gene therapy, and immunotherapy in the fields of rare diseases, cancer, and cardiovascular diseases. “We will also see many more vaccines based on the wonderful mRNA technology [the tech behind the novel COVID vaccines]. I believe all current vaccines will become mRNA-based. In addition, we will see the development of vaccines against diseases for which we currently have none, such as HIV and different types of cancer. This will take more work, but it is super exciting.”
To significantly boost the development of these therapies and make a real-world impact, Barzel heads his lab’s collaboration with the Tel Aviv Sourasky Medical Center as part of the joint Dotan Center for Advanced Therapies. “This collaboration is crucial for our ability to take our ideas from the bench to the bedside,” he explains. The partnership fast-tracks the process of working with patient samples, allowing the researchers to get efficient results quickly and establishing an atmosphere of cooperation that translates into real progress in the clinic.
Source: TAU Review





